Key Facts
Page Updated 2-9-26
- Agent: Measles (rubeola) virus
- Reservoir/Source: Human/Respiratory tract secretions and fomites.
- Symptoms: Consider measles in patients of any age who have a fever ≥101 F, plus at least one of the 3 “Cs” (cough, coryza, or conjunctivitis) and a descending rash that starts on the face. The rash typically follows the onset of illness within 4 days. Atypical and modified rash can start on hands and feet.
- Complications: Diarrhea, otitis media, pneumonia, encephalitis, subacute sclerosing panencephalitis, acute disseminated encephalomyelitis, keratitis, myocarditis, pericarditis, and death.
- Differential Diagnoses: Kawasaki, rubella, scarlet fever, enteroviruses, and other febrile rash exanthems.
- Incubation: From exposure to rash onset, 7-21 days with an average of 14 days.
- Communicability and Transmission: From 4 days before onset of rash to 4 days after its appearance via direct contact with infectious droplets or by airborne spread.
- Presumption of Immunity Criteria: See Box 2.
- Specimen Collection/Lab Testing: Throat or nasopharyngeal (NP) swab, urine, and serum, see Box 1 for details.
- Specific Treatment: Supportive care only; antiviral agent not available. Vitamin A supplementation may be beneficial for reducing measles severity and risk of complications.
- Post-Exposure Prophylaxis: MMR or immune-globulin/intramuscular immune globulin (IMIG). See “Investigation of Asymptomatic Contacts to a Measles Case” for more details.
- Recommended Vaccines: MMR and MMRV
- Reportable Criteria: Report by phone immediately upon suspicion of measles: 213-351-7800 weekdays 8:30am - 5:00pm, or 213-974-1234 after hours.
Do not wait for laboratory confirmation before reporting.
Investigation of a Suspect Measles Case
Investigation of Asymptomatic Contacts to a Measles Case
Boxes, Appendix, & Resources
Box 1: Measles Specimen Collection & Lab Testing
Collect all specimens in consultation with VPDC. Real-time polymerase chain reaction (RT-PCR) testing can be done at the Public Health Lab or certain commercial labs.
Testing for Patients with a Rash
- Throat or NP swab for RT-PCR
- Timeline: Collect within 2 weeks of rash onset.
- Equipment
- Use sterile synthetic (non-organic) swab (e.g., Dacron)
- Place swab after collection into liquid viral or universal transport medium.
- Urine for RT-PCR
- Timeline: Collect within 2 weeks of rash onset
- Equipment:
- Collect 10-50 ml urine in a sterile container from the first part of the urine stream. The first morning void is ideal.
- Serum for IgM test for recent infection or recent
vaccination
- Timeline: Acute serum optimally collected at least 72 hours after rash onset
- Equipment:
- Collect 7-10 ml of blood in a serum separator tube (SST, red-gray rubber stopper or gold plastic stopper).
- If acute IgM is negative, a convalescent serum specimen may be requested 14-28 days later.
Immunity Testing for Asymptomatic Patients
- Serum for IgG only
- Equipment is same as IgM serum test
Storage and Lab Requisition Forms
- Call the Public Health Lab courier to pick-up specimens: 562-658-1460 (M-F 8AM-5PM).
- Lab forms for testing symptomatic patients (not required if orders are submitted via ORCHID)
- Lab forms for testing asymptomatic patients
- Store specimens at 4°C/39°F until pick-up and ship cold (not directly in contact with frozen icepacks). If unable to ship within 48 hours, freeze specimen immediately at -70°C (except for urine – centrifuge and store at 4°C).
Box 2: Presumption of Immunity Criteria
Contacts may be presumed immune if they meet any of the following criteria:
High Risk
- Documentation of two doses of measles vaccine given 1968 or later, separated by at least 28 days, with the first dose on or after first birthday
- A positive IgG test for measles
- Laboratory confirmation of previous disease
Low Risk
- Any high-risk presumptive immunity criteria.
- Born prior to 1957
- Born in any country (including the U.S.) in 1976 or later AND attended a U.S. primary or secondary school*
- Have written documentation with date of receipt of at least one dose of measles vaccine given on or after first birthday in 1968 or later
- Served in the U.S. armed forces
- Entered the U.S. as a permanent U.S. resident or became one in 1996 or later (i.e., have a “green card”)*
*Unless known to be unvaccinated for measles, e.g., having a medical contraindication to vaccination or being philosophically or religiously opposed to vaccinations.
Box 3: Severely Immunocompromised Criteria
Severely immunocompromised patients who are exposed to measles should receive IGIV prophylaxis regardless of immunologic or vaccination status because they might not be protected by the vaccine. Per IDSA, persons with high-level immunosuppression include those:
- With combined primary immunodeficiency disorder (e.g., severe combined immunodeficiency);
- Who are receiving cancer chemotherapy;
- On treatment for ALL within and until at least 6 months after completion of immunosuppressive chemotherapy;
- Within 2 months after solid organ transplantation;
- Who have received a bone marrow transplant until at least 12 months after finishing all immunosuppressive treatment, or longer in patients who have developed graft-versus-host disease;
- With HIV infection with a CD4 T-lymphocyte count <200 cells/mm3 (age >5 years) and percentage <15 (all ages) (some experts include HIV-infected persons who lack recent confirmation of immunologic status or measles immunity);
- Receiving daily corticosteroid therapy with a dose ≥20 mg (or >2 mg/kg/day for patients who weigh <10 kg) of prednisone or equivalent for ≥14 days; and
- Receiving certain biologic immune modulators, that is, a tumor necrosis factor-alpha (TNF-α) blocker or rituximab.
After HSCT, duration of high-level immunosuppression is highly variable and depends on type of transplant (longer for allogeneic than for autologous), type of donor and stem cell source, and post-transplant complications such as graft vs host disease (GVHD) and their treatments.
Figure 1: Measles Contact Management Algorithm
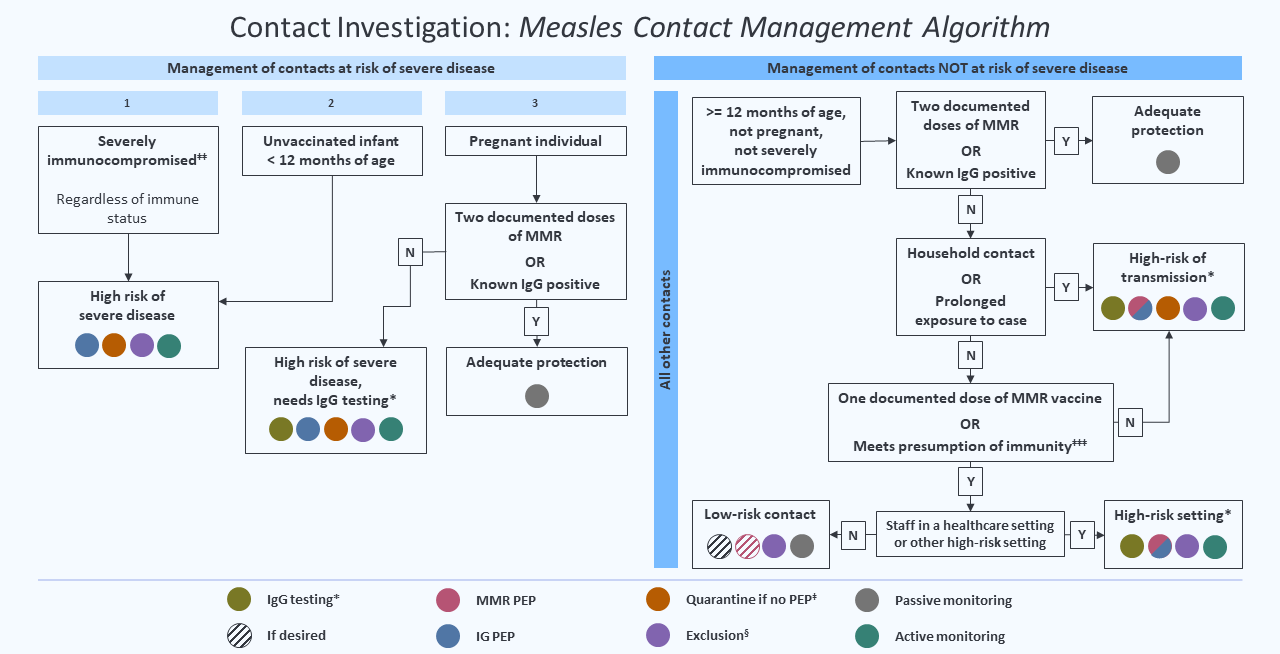
* For measles contacts who have tested measles IgG negative or equivocal in a commercial lab, VPDC should be consulted regarding potential retesting at DPH PHL. If a contact tests positive for IgG at PHL or a commercial lab, consider them to be immune.
‡ Implement quarantine from day 7 after first exposure through day 21 after last exposure. If symptoms consistent with measles develop, the exposed person should be isolated and tested.
§ Exclude from high-risk settings (e.g., childcare facilities with infants and healthcare facilities; see definition above) from day 7 (day 5 for healthcare workers in healthcare settings) after first exposure through day 21 after last exposure. Those who have received IG should exclude through day 28 after last exposure.
‡‡ If it can be done rapidly, it is recommended that pregnant persons be tested for measles IgG prior to administering IGIV if it is likely that they have received vaccine or had disease. If an exposed pregnant person is IgG negative or IgG equivocal or has unknown status and IgG test results (or retest at VRDL) will not be known by day 6 after exposure, administer IGIV.
‡‡‡ A self-reported history of measles disease without documentation is not
acceptable as a presumption of immunity. See Box 2 for presumption of immunity criteria. If a low-risk contact has a measles IgG negative or IgG equivocal result, and subsequently provides documentation of two doses of MMR vaccine, base public health decisions on the two documented doses of MMR vaccine, i.e., presume immunity.
Figure 2:
Figure 2: Immune Globulin (IG) Dosage for Measles Exposure
| Indications |
Dose |
Interval before MMR vaccine administration |
|---|---|---|
Infants <12months of age |
0.5 ml/kg IM (max dose = 15mL) |
6 months |
| Susceptible immunocompetent contacts <30 kg/66lbs5 |
0.5 ml/kg IM (max dose =15mL) |
6 months |
| Pregnant women without evidence of immunity |
400 mg/kg IV (intravenously) |
8 months and nonpregnant |
| Severely immunocompromised persons6 |
400 mg/kg IV (intravenously) |
8 months |
1IMIG should be administered at room temperature and within 6 days of exposure.
2IG should be administered to susceptible infants and children <30 kg and high-risk persons (pregnant women and severely immunocompromised
persons).
3IMIG can be given to any person <30 kg who lacks evidence of measles immunity, but priority should be given to persons exposed in settings with intense, prolonged, close contact (e.g., household, child care, classroom, etc.) or persons who are more likely to develop severe measles (infants, immunocompromised children).
4The maximum intramuscular dose of IG is 15 ml for all persons.
5Persons weighing >30 kg/66 lbs are unlikely to receive an adequate amount of measles antibody from IMIG.
6See Box 3.
Table 1: Follow-Up of High-Risk Measles Contacts
| High-risk contacts (persons with potential for severe illness if infected or to whom the transmission potential is high) | IgG testing* |
PEP† | Quarantine if no PEP‡ | Exclusion | Monitoring§ |
|---|---|---|---|---|---|
| Unvaccinated infants <6 months of age | No | IG only | Yes | Yes** | Active |
| Unvaccinated infants 6-11 months of age†† | No | MMR or IG‡‡ | Yes | Yes** | Active |
| Pregnant persons without 2 documented MMR vaccine doses or serologic evidence of immunity‡‡ | Yes* | IG only | Yes | Yes** | Active |
| Severely immunocompromised§§ | No | IG only | Yes | Yes** | Active |
| Household contact or contact with prolonged exposure without 2 documented MMR vaccine doses or serologic evidence of immunity | Yes* | MMR or IG*** | Yes | Yes** | Active |
| All other immunocompetent contacts with 2 documented MMR vaccine doses or serologic evidence of immunity | No | No | No | No | Passive |
* For measles contacts who have tested measles IgG negative or equivocal in a commercial lab, VPDC should be consulted regarding potential retesting at DPH PHL. If a contact tests positive for IgG at PHL or a commercial lab, consider them to be immune.
† Contacts at high risk of severe infection (severely immunocompromised people, unvaccinated infants, and susceptible pregnant persons) should receive IG PEP within 6 days or less from the date of last exposure to measles.
‡ Implement quarantine from day 7 after first exposure through day 21 after last exposure. If symptoms consistent with measles develop, the exposed person should be isolated and tested.
§ Exclude from high-risk settings (e.g., childcare facilities with infants and healthcare facilities; see definition above) from day 7 (day 5 for healthcare workers in healthcare settings) after first exposure through day 21 after last exposure. Those who have received IG should exclude through day 28 after last exposure.
** Exclude from high-risk settings (e.g., childcare facilities with infants and healthcare facilities; see definition above) from day 7 (day 5 for healthcare workers in healthcare settings) after first exposure through day 21 after last exposure. Those who have received IG should exclude through day 28 after last exposure.
†† MMR vaccine can be given as PEP within 72 hours or less from the time of exposure to persons >6 months of age who do not have contraindications for MMR vaccine. IMIG can be given as PEP for exposed infants <12 months of age <6 days from exposure. Persons >12 months of age who may have been vaccinated or had disease and receive MMR vaccine as PEP should have blood drawn and tested for measles IgG if measles IgG status is unknown at the time of MMR administration.
‡‡ If it can be done rapidly, it is recommended that pregnant persons be tested for measles IgG prior to administering IGIV if it is likely that they have received vaccine or had disease. If an exposed pregnant person is IgG negative or IgG equivocal or has unknown status and IgG test results (or retest at VRDL) will not be known by day 6 after exposure, administer IGIV.
§§ Refer to
IDSA guidance for high-level immunosuppression criteria.
*** IMIG can be considered for susceptible persons in this category weighing <30 kg (<66 pounds). There is no recommendation for IGIM in susceptible persons >30 kg (≥66 pounds). MMR PEP is preferred if <72 hours of exposure. IGIV is not recommended for low-risk contacts weighing ≥30 kg (≥66 pounds).
Table 2: Follow-Up of Measles Contacts Who Work in A High-Risk Setting
| Contacts who work in a healthcare setting or other high-risk setting | IgG testing* | PEP | Quarantine if no PEP‡ | Exclusion | Monitoring |
|---|---|---|---|---|---|
| High-risk for severe disease due to personal medical history and without 2 documented MMR vaccine doses or serologic evidence of immunity |
See Table 1 | ||||
| Low risk for severe disease and with 1 documented MMR vaccine dose and no serologic evidence of immunity |
Yes | MMR | No | Yes** | Active |
| Low risk for severe disease and with
no documented MMR vaccine doses and no serologic evidence of immunity |
Yes | MMR | Yes | Yes** | Active |
| With 2 documented MMR vaccine doses or serologic evidence of immunity |
No | No | No | No | Passive |
* For measles contacts who have tested measles IgG negative or equivocal in a commercial lab, VPDC should be consulted regarding potential retesting at DPH PHL. If a contact tests positive for IgG at PHL or a commercial lab, consider them to be immune.
** Exclude from high-risk settings (e.g., childcare facilities with infants and healthcare facilities; see definition above) from day 7 (day 5 for healthcare workers in healthcare settings) after first exposure through day 21 after last exposure. Those who have received IG should exclude through day 28 after last exposure.
Table 3: Follow-Up of Low-Risk Measles Contacts in a Low-Risk Setting
| Low-risk contacts
(immunocompetent persons, persons >12 months of age, not pregnant, not a healthcare worker, not a household contact) |
IgG testing* | PEP | Quarantine if no PEP‡ | Exclusion | Monitoring§ |
|---|---|---|---|---|---|
| Two documented doses of MMR vaccine (3% will be susceptible) | No | No | No | No | Passive |
| Known to be measles IgG positive (<1% will be susceptible) | |||||
| Meets presumption of immunity criteria (including 1 documented MMR dose) | If desired | MMR if desired | No | Yes** | Passive |
| Unknown or no documentation of vaccination or immune status, without presumption of immunity††† |
Yes* | MMR | Yes | Yes** | Active |
| Known to be unvaccinated††† | No | MMR | Yes | Yes** | Active |
* For measles contacts who have tested measles IgG negative or equivocal in a commercial lab, VPDC should be consulted regarding potential retesting at DPH PHL. If a contact tests positive for IgG at PHL or a commercial lab, consider them to be immune.
‡ Implement quarantine from day 7 after first exposure through day 21 after last exposure. If symptoms consistent with measles develop, the exposed person should be isolated and tested.
§ Exclude from high-risk settings (e.g., childcare facilities with infants and healthcare facilities; see definition above) from day 7 (day 5 for healthcare workers in healthcare settings) after first exposure through day 21 after last exposure. Those who have received IG should exclude through day 28 after last exposure.
** Exclude from high-risk settings (e.g., childcare facilities with infants and healthcare facilities; see definition above) from day 7 (day 5 for healthcare workers in healthcare settings) after first exposure through day 21 after last exposure. Those who have received IG should exclude through day 28 after last exposure.
††† See Box 2 for “Presumption of Immunity Criteria for Low-Risk Contacts”. A self-reported history of measles disease without documentation is not acceptable as a presumption of immunity. If a low-risk contact has a measles IgG negative or IgG equivocal result, and subsequently provides documentation of two doses of MMR vaccine, base public health decisions on the two documented doses of MMR vaccine, i.e., presume immunity.
Resources
Measles Notification Letters and Flyers:
- Exposure Notification for Individuals Exposed to Measles:
- Facility Notification of Measles Exposure: English | Spanish
- Measles Exposure Notification Flyer: English | Spanish | Chinese Simplified | Chinese Traditional | Armenian | Vietnamese
- General Notification After Confirmed Measles Cases: English | Spanish
- General Notification of the end of Measles Situation
Measles Case Investigation Forms and Templates:
- Measles Case Isolation Letter
- Off Work Notice (DPH Only)
- Measles Activity Log
- Initial Assessment Form
- Measles Line List
Measles Guidance and Protocols:
- Exanthems – Differential Diagnosis
- PROTOCOL: Intramuscular Immune Globulin (IMIG) Gamastan S/D Administration for Susceptible Measles Contacts (DPH Only)
- CDPH Measles, Mumps, Rubella, and Varicella vaccine (MMRV) Recommendations
- CDPH Measles Investigation Quicksheet
- CDPH Measles Healthcare Exposure Investigation Quicksheet
Virtual Trainings:
- Recap: Measles Contact Investigation - IRIS Documentation Training (05/06/2025)
- IRIS Job Aids (DPH Only)
- IMIG/IGIM for Measles PEP (DPH Only, 08/06/2024)
Acute Communicable Disease Control Manual (B73):

