Outpatient Treatments for COVID-19
Three antiviral therapies are currently FDA approved or authorized for outpatient treatment of COVID-19 infection and recommended by the NIH COVID-19 Treatment Guidelines Panel. They are not approved for pre- or post-exposure prophylaxis. Importantly, they retain full activity against the current variant mix in California.
Note: The FDA has removed the requirement for a positive SARS-CoV-2 viral test in order to prescribe these medications. Although the agency still strongly recommends the use of viral testing to help diagnose COVID-19 this change allows for providers to consider treatment in the rare instances where an individual with a known exposure who develops signs and symptoms consistent with COVID-19 presents with a negative test. See FDA product specific FAQs for more information.
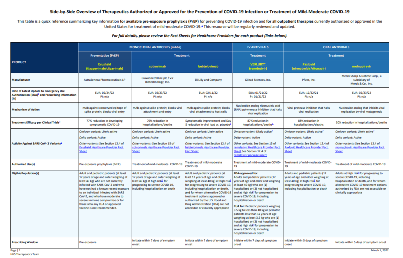
| Antiviral Medication (in order of preference) | FDA Status | Mode/duration of Administration | Start time from symptom onset | Age/Weight | Fact sheets/FAQs |
|---|---|---|---|---|---|
| 1. Paxlovid | Approved (adults) EUA (age 12-17) | Oral twice daily for 5 days | ≤5 days | Age ≥12 who weigh ≥40kg | Providers Approved label EUA fact sheet Patient/caregivers FAQs |
| 2. Remdesivir | Approved | IV infusion daily for 3 days | ≤7 days | Adults and children age ≥28 days who weigh ≥3kg | Providers Patients FAQs |
| 3. Molnupiravir | EUA | Oral twice daily for 5 days | ≤5 days | Adults ≥18 (not recommended in pregnancy) | Providers Patient/caregivers FAQs |
Paxlovid
Paxlovid (nirmatrelvir tablets and ritonavir tablets, co-packaged for oral use), given within 5 days of symptom onset, is the preferred treatment for adults and pediatric patients (12 years of age and older weighing at least 40 kg) with symptomatic COVID-19 and risk factors for progression. It is taken orally twice a day for 5 days.
See the FDA Paxlovid approved label/fact sheet (EUA) for detailed prescribing information including.
Most commonly used medications can be safely co-administered with Paxlovid
- Paxlovid can alter the concentrations of other drugs, so it is important to assess for potential drug-drug interactions.
- See NIH guidance Drug-Drug Interactions Between Ritonavir-Boosted Nirmatrelvir (Paxlovid) and Concomitant Medications and the Liverpool COVID-19 Drug Interactions website
- The Infectious Disease Society of America offers guidance on simple steps that can be taken to avoid significant interactions with commonly prescribed medications, such as brief suspension or dose reduction.
HHS Paxlovid website includes prescribing information, FAQs, and other resources for providers and patients.
Paxlovid Patient Assistance Program (PAP):
- Through December 31, 2024, individuals covered under federal programs, such as Medicare or Medicaid, and uninsured patients are eligible for the PAP and can receive Paxlovid at no cost.
- This includes all patients publicly insured through Medicare (with or without Part D, Part B, or Part C and inclusive of Medicare Advantage), Medicaid/CHIP, TRICARE, and patients insured through the VA Community Care Network.
- Participating PAP dispensing sites will be reimbursed for any product dispensed, along with a dispensing fee.
- Retail pharmacies that would like to learn more about participating in the USG PAP can email the program vendor at PharmacyNetworkContract102101@assistrx.com.
- Concurrently, Pfizer is operating a Paxlovid Co-Pay Savings Program for eligible privately (commercially) insured patients.
- The Co-Pay program is accessible through Paxlovid.com for patients and Paxlovid.pfizerpro.com for health care providers.
FDA: Letter of authorization | FAQs on the EUA | FDA letter: new medication packaging for patients with moderate renal impairment | Fact sheet for health providers | Fact sheet for patient/caregivers
Other resources: Pharmacist Authority to Prescribe
Remdesivir
Remdesivir (Veklury) is an antiviral drug approved by the FDA for treatment of adults and pediatric patients (28 days of age and older weighing at least 3kg). It is given as an IV infusion for a total of 3 days initiated as soon as possible and within 7 days of symptom onset.
Given the emergence of variants resistant to mAbs, providing remdesivir in the outpatient setting may be of particular importance for facilities and providers that see large numbers of immunosuppressed patients such as transplant and oncology centers, or high-risk pediatric populations. Providers are encouraged to order remdesivir to have on hand for patients unable to take Paxlovid.
Availability of Veklury
Hospital ordering process:
- AmerisourceBergen Specialty Distribution: 1-800-746-6273 or email remdesivir@amerisourcebergen.com
- Cardinal Specialty: 1-855-855-0708
- McKesson Plasma: 1-877-625-2566
Non-Hospital ordering process:
- AmerisourceBergen Specialty: 1-800-746-6273 or C19therapies@AmerisourceBergen.com
- Cardinal Specialty: 1-855-855-0708 or GMB-SPD-CSORDERENTRY@cardinalhealth.com
- Therapeutics locator: HHS is asking providers who administer outpatient Veklury (remdesivir) to add their infusion site to the COVID-19 Therapeutics Locator in order to improve public awareness and product access. Infusion sites can learn more and opt into the therapeutics locator here.
- CMS has created the Healthcare Common Procedure Coding System (HCPCS) code J0248 for reimbursement when administered in an outpatient setting. This code is available for use by all payers and is effective for dates of service on or after December 23, 2021. Additional information is available here.
FDA:
Prescribing information for adults and children
Information for providers
vekluryhcp.com
| Information for patients
www.veklury.com
Molnupiravir (Lagevrio)
Molnupiravir (Lagevrio) is authorized as an alternative therapy when Paxlovid and remdesivir are not available, feasible to use, or clinically appropriate. It is given as 800 mg (four 200 mg capsules) taken orally every 12 hours for 5 days. Treatment started as soon as possible after diagnosis of COVID-19 and within 5 days of symptom onset.
- Molnupiravir is a nucleoside analogue that inhibits SARS-CoV-2 replication by viral mutagenesis.
- Guidance is now available on the provider fact sheet for the preparation and administration of molnupiravir via nasogastric and orogastric tube.
- Several studies have shown a risk reduction in severe disease after molnupiravir treatment with the most significant benefit in those at highest risk or elderly (≥75 years of age). In addition, there is evidence of more rapid symptom resolution and viral load reductions in people treated with molnupiravir. See table below.
- Use in adults 18 and older only. Not recommended in pregnancy. If the provider and patient choose to use the product during pregnancy, it is advised that the patient be registered in the Merck Pregnancy Surveillance Program.
- Providers should carefully review the Fact sheet for health providers before prescribing to ensure that the patient’s condition warrants treatment, that there are no drug interactions, and that there are contraindications to therapy.
Availability of Molnupiravir
- EUA-labeled Molnupiravir is active and available to order for pick up through the weekly order forms emailed to in-network providers every Friday morning.
- For commercial product, please visit www.merckorders.com or call the Merck Order Management Center at 800-MERCK-RX (800-637-2579).
Lagevrio Patient Assistance Program:
- The Merck Patient Assistance Program (a 501c3 non-profit organization) provides Lagevrio free of charge to patients who meet its eligibility criteria and who, without assistance, could not otherwise afford the product.
- This product is ONLY available through an URGENT NEED request. Health Care Providers must call 800-727-5400 and tell the program representative that they are making an Urgent Need Request for LAGEVRIO.
- You may also check eligibility on MerckHelps.
- Merck is also operating a Lagevrio Co-Pay Savings Program. Through this program, eligible privately insured patients may save on their out-of-pocket costs for their prescriptions for Lagevrio.
FDA: Letter of authorization | FAQs on the EUA | Fact sheet for health providers | Fact sheet for patient/caregivers
Procuring medication for your patients
- If you are interested in procuring free USG supplied Molnupiravir as you continue to build out your transition plans to the commercial market, please contact DPH-Therapeutics@ph.lacounty.gov. If you already receive weekly order forms from the DPH therapeutics team you can also request free USG doses of Molnupiravir only through that process. We will continue to support the provider community while our supplies last. Providers will have to attest that doses will be offered free of charge and that they are not already receiving free doses from other government sources. As of March 8th, 2024 Paxlovid is only available for commercial purchase.
- Commercial products can be obtained through your preferred wholesale distributor.
- This information is intended for medical providers. Information for the public is available here.
- Patients who are unable to access oral COVID-19 therapeutics through their own provider or who do not have health insurance can be referred to a Test to Treat program or the DPH Public Health Call Center/Tele-Health service, which provides testing, consultation, and prescriptions. 1-833-540-0473 - open 7 days a week, 10:00 am-6:00 pm.