IRB Application Information
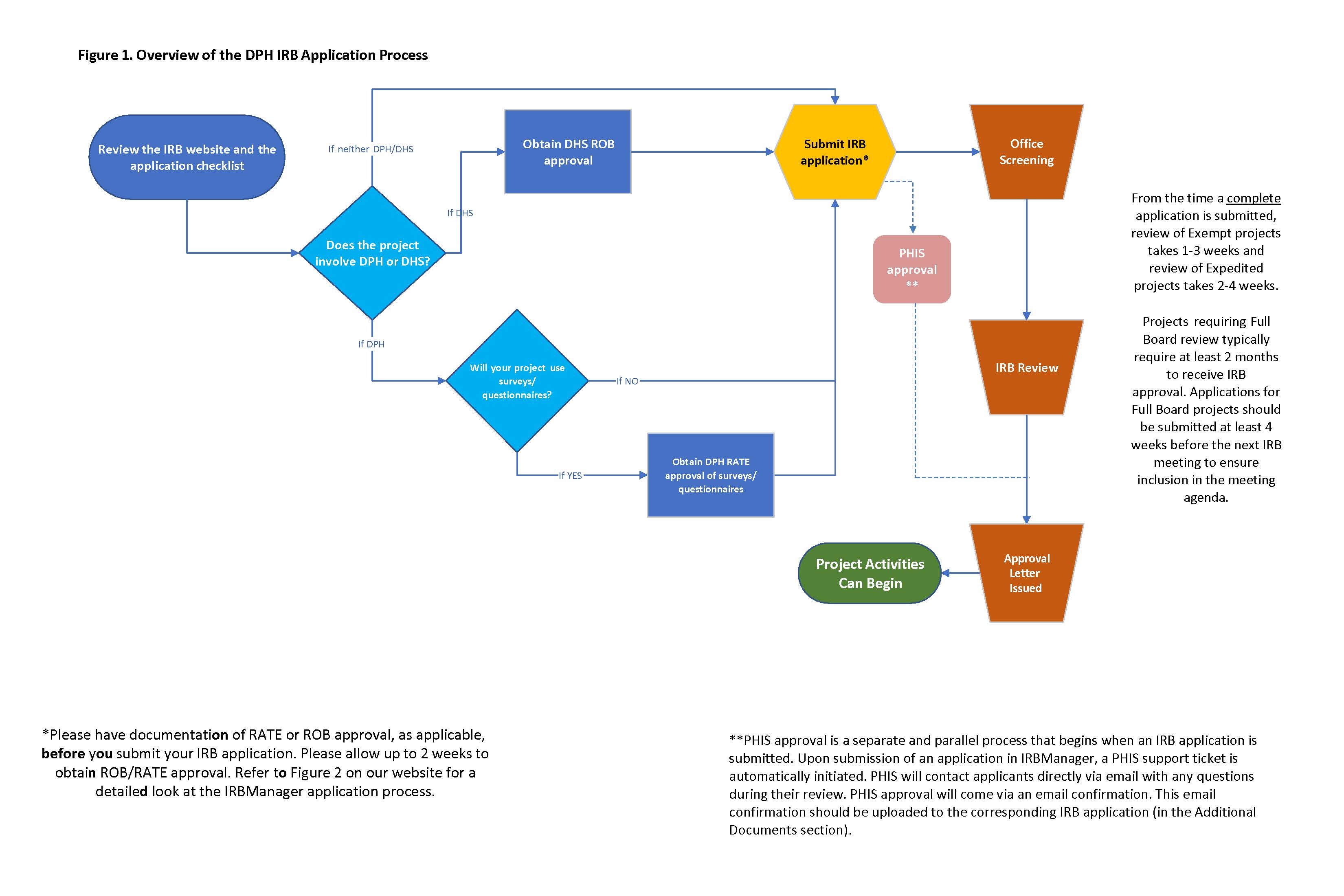
Overview of the IRB application process
Please review the information below before submitting an IRB application for review.
All projects seeking IRB review must submit an application using a web-based application called IRBManager. Visit the IRBManager page when you are ready to submit an application. If you are not sure whether your project needs IRB review, please contact the Office of the IRB via email at IRB@ph.lacounty.gov for clarification.
Project activities CANNOT begin until a formal approval letter from the Office of the IRB has been obtained.
If you would like to submit a new application for IRB review, review the New IRB Application section below and familiarize yourself with the new application checklist as well as requirements for RATE review of surveys (for DPH projects) and DHS ROB approval (for DHS projects).
If you need to make any changes to a project that has already received IRB approval, including adding/removing personnel, please submit an amendment application (refer to the Amendments section below). Any changes to IRB-approved projects should not be implemented until the IRB approves an amendment application for the proposed changes.
Annual progress reports are required for all projects and must also be submitted in IRBManager (refer to the Annual Progress Reports section below). Some projects may be required to submit Continuing Review requests instead of progress reports as noted in the original approval letter (refer to the Continuing Review section below).
Click on the image to view an overview of the steps involved in the IRB application process:
What projects does the IRB review?
The DPH IRB reviews projects involving clients/patients, staff, facilities, and/or funding from the Department of Public Health (DPH), the Department of Health Services (DHS) (excluding the hospitals), and certain community-based organizations. A project is defined as the collection or analysis of data from or about human subjects, including but not limited to the following activities:
- Focus groups
- Semi-structured interviews
- Data abstraction from medical records
- Surveys
*IRB procedures allow exemption for some projects that are classified as routine public health activity rather than research or related activities. Please consult with the Office of the IRB for a determination.
Public Health Surveillance and Public Health Practice
Please refer to the the following document regarding IRB review of activities that can be considered public health surveillance and activities that can be considered public health practice: Public Health Surveillance and Practice
Not Subjects Research Determinations
Please consult with the Office of the IRB before submitting a Not Human Subjects Research application. The Not Human Subjects Research application is not the appropriate application for projects that the IRB deems "Exempt as Non research".
New IRB application
Review the checklist below to make sure you have all items necessary to submit a complete IRB application. An application cannot undergo IRB review until it is screened and deemed complete.
 Checklist for New Applications
Checklist for New Applications
IRB applications undergo a two-phase process: 1) Screening, and 2) Review.
Phase 1 - IRB Screening
This phase involves IRB staff performing an administrative check to make sure the application is complete, including required approvals and documents, as described in the New IRB application checklist below. If any issues are identified, IRB staff will return the application to the applicant with an itemized request for changes and/or additional information. The application should be re-submitted with the requested changes. IRB staff will screen the application again; this will repeat until an application is deemed complete. An application cannot proceed to the Review phase until it is deemed complete.
Phase 2 – IRB Review
Once an application is complete, it is assigned to a reviewer who reviews the application and supporting materials to ensure the proposed activities meet the ethical and quality standards outlined in the code of federal regulations (45 CFR 46, known as "the Common Rule") as well as DPH IRB Standards of Practice/policies. A reviewer may identify issues (referred to as "stipulations") that need to be addressed, in which case the application will be returned to the applicant so that changes can be made. The application will need to be re-submitted with the stipulations addressed.
Project activities CANNOT begin until a formal approval letter from the Office of the IRB has been obtained.
How long does the process take?
Prior to IRB submission:
Projects involving DHS (including patients, staff, facilities, data, and funding) must obtain approval by the DHS Research Oversight Board (ROB). ROB review must be completed prior to submitting an application for IRB review. Please allow at least two weeks to obtain ROB review. Instructions on how to comply with the ROB review requirement are located in the application checklist (above) and also in the section below.
From the time a complete application is received by the IRB:
- Review of exempt projects takes on average 1-3 weeks.
- Review of expedited projects take on average 2-4 weeks.
- Projects requiring Full Board review typically require at least 2 months to receive IRB approval. Applications for Full Board projects should be submitted at least 4 weeks before the date of the following IRB meeting to ensure inclusion in the meeting agenda.
- Please note: estimated turnaround times depend on how long the applicant takes to respond to IRB requests for changes and/or additional information.
DPH RATE survey policy/DHS ROB
DPH RATE Review
We are no longer requiring surveys to be reviewed by Office of Health Assessment and Epidemiology (OHAE) Rapid Assessment, Training and Evaluation unit (RATE). Please ensure your survey has been reviewed and approved by program leadership and subject matter experts in your program. If your program does not have this expertise and you would like help at any point in the creation of your survey, please contact OHAE Director, Dr. Megha Shah, who will determine the best team in OHAE to assist you. Your survey and project may need IRB approval if it involves research, evaluation, needs assessment or certain public health surveillance. If you have questions about whether or not your project needs IRB approval, please contact the IRB office at: irb@ph.lacounty.gov.
DHS ROB Review
If your project involves DHS (excluding the hospitals), you will need to include with your IRB application documentation that the project has been reviewed and assigned a priority category by DHS’ Research Oversight Board (ROB). To comply with the ROB requirement, please submit all project materials including a protocol, budget and any data collection instruments to irb@ph.lacounty.gov and IRB staff will forward it to the ROB for review. The requestor will be contacted via email with the results of the ROB review. Save this email confirmation as a PDF document and upload it to your IRB application.
PHIS and DHS Information Security Office Approval
PHIS Information Security Office Approval
PHIS Information Security Office (ISO) approval is only required for DPH projects that satisfy ONE or BOTH of the following conditions:
- project involves outside contractors who will be collecting/accessing Personally Identifiable Information (PII)/ Protected Health Information (PHI)
- project involves use of devices and/or software that was not installed/approved by DPH IT
You will be asked on the IRB application to indicate whether either of the two conditions above apply to your project. If you answer "Yes" to either condition, you will be prompted to respond to a set of information security questions in the IRB application. Once your application is submitted, a support ticket will automatically be opened with PHIS. PHIS staff will review your responses to the information security questions and they will contact you directly via email with any questions. PHIS ISO approval will be sent via email; a copy of the email confirming approval should be saved as a PDF and included with the IRB application.
DHS Information Security Approval
For DHS projects, applicants should contact DHS' Information Security Officer, Vahe Haratounian, at vharatounian@dhs.lacounty.gov to obtain security approval. A copy of the email from Vahe confirming approval should be saved as a PDF and included with the IRB application.
Adverse/Reportable Events
Any adverse/reportable events or deviations from approved protocols must be reported to the Office of the IRB no later than 7 days after the research team has been made aware of the event in question. If you are project staff and need to report an adverse/reportable event/protocol deviation, please submit an adverse/reportable events form in IRBManager. Refer to Section 12 of the IRBManager user guide for assistance with completing this form.
Annual Progress Reports
An annual progress report is due for all projects even if they do not have an expiration date. Progress reports must be submitted through IRBManager. Any project that does not submit an annual progress report at least 2 weeks before the due date (as stated on the most recent approval letter) will be automatically closed by the system and a new application will need to be submitted in order to re-open the project. Please contact the Office of the IRB if you anticipate any challenges in meeting due dates. Refer to Section 6 of the IRBManager user guide for instructions on how to submit a progress report application.
Amendments
Please submit an amendment request in IRBManager PRIOR to implementing any changes to previously approved activities and/or materials, including the protocol, data collection instruments, or personnel. PI/project lead changes must be submitted as amendments and the current PI/project lead must provide their signature on the application before it can be approved.
IMPORTANT: If the PI or co-PI plan to end/change their institutional affiliation, an amendment application should be submitted BEFORE the date of the change in affiliation to ensure access to the institutional email address on file (notifications for providing signatures will be sent to this email address).
Review the checklist below to make sure you have all the items necessary to submit a complete amendment application.
When you are ready to submit your amendment application, refer to Section 6 of the IRBManager user guide for instructions on how to submit an amendment application.
 Checklist for Amendment Applications
Checklist for Amendment Applications
Note: amendments may not be submitted with annual continuing review requests; please submit these applications separately.
Continuing Review
A request for continuing review must be approved for:
- all full board projects and
- any projects that are given an expiration date by the IRB (expiration date is noted on the approval letter).
If continuing review is required, as stated in the approval letter, please submit a continuing review application in IRBManager. Refer to Section 6 of the IRBManager user guide for instructions on how to submit a Continuing Review application.