
Key Facts
Page Updated 1-27-26
- Agent: Varicella-zoster virus (VZV), a member of the herpesvirus family. VZV infection causes three clinically distinct forms of disease: varicella (chickenpox), herpes zoster (shingles), and congenital varicella syndrome.
- Reservoir/Source: Human/Mucous membranes and vesicles.
- Symptoms:
Primary varicella (chickenpox): This is a person’s first infection with VZV.
Typical disease in unvaccinated individuals: In children, varicella lesions are often the first sign of disease. In adults and some children, patients may have 1-2 days of fever and malaise prior to rash onset.
- The rash may first appear on the chest, back, and face and then spreads over the entire body. This can include inside the mouth, on the eyelids, and/or on the genital area.
- Patients have crops of red bumps that turn into itchy, fluid-filled blisters which eventually become scabs, usually in five to seven days.
"Breakthrough" varicella: Infection occurring in a vaccinated person more than 42 days after vaccination.
- Rash is usually mild with fewer than 50 skin lesions and patients do not often experience fever.
- Illness is shorter in duration compared to unvaccinated people who get primary varicella.
Perinatal/neonatal varicella: Occurs within first 10 days of life from a mother infected from 5 days before to 2 days after delivery.
- Neonates may present with fever, followed by a generalized vesicular eruption. It can disseminate quickly and cause life threatening complications.
- Prodrome of pain, itching, or tingling in the area where the rash will develop.
- Development of a vesicular, often painful rash in 1-2 adjacent dermatomes (does not cross midline); most commonly appears on the trunk along a thoracic dermatome or on the face.
- Older adults or those with weakened immune systems are more likely to have atypical presentation or dissemination of VZV to other dermatomes and organs (brain, optic nerve, liver, lungs) as well as complications including postherpetic neuralgia.
- Complications:
Primary varicella (chickenpox):- Bacterial infections of the skin and soft tissues, including Group A streptococcal infections which can lead to myositis, necrotizing fasciitis, and toxic shock syndrome
- Bleeding problems (hemorrhagic complications, thrombocytopenia)
- Sepsis, pneumonia, encephalitis
- Hepatitis, pericarditis, glomerulonephritis, orchitis
- Perinatal/Neonatal disease has a high rate (20-50%) of dissemination with a 20-30% fatality rate. The severity of disease results from fetal exposure to the virus without the benefit of passive maternal antibody. Postnatally acquired varicella occurs after 10 days of age and is rarely fatal.
- Postherpetic neuralgia
- Dissemination resulting in hearing or vision loss, encephalitis, pneumonia or death
- Differential Diagnoses: Generalized herpes simplex, impetigo, drug rash, MPOX, secondary syphilis, smallpox, and other viral exanthems.
- Incubation: From exposure to rash onset, 10-21 days with an average of 14-16 days. May be prolonged after receipt of varicella zoster immune globulin (VariZIG) and in people who are severely immunocompromised (see Box 3).
- Communicability and Transmission:
- Primary varicella is highly contagious. The virus can be spread from person to person by direct contact; inhalation of aerosols from vesicular fluid of skin lesions of primary varicella or herpes zoster; and possibly through infected respiratory secretions that could have aerosolized.
- Patients are contagious 1–2 days before rash onset until all lesions have crusted (usually about 5 days).
- Vaccinated people may develop lesions that do not crust. These people are considered contagious until no new lesions have appeared for 24 hours.
- People who have never had chickenpox and have never been vaccinated against it can get infected with VZV by having direct contact with active shingles lesions. They will develop primary varicella, not shingles.
- Presumption of Immunity Criteria: See Box 2.
- Specimen Collection/Lab Testing: See Box 1.
- Specific Treatment: Acyclovir or valacyclovir administered to susceptible individuals is effective in reducing varicella morbidity when administered within 24 hours of rash onset.
- Post-Exposure Prophylaxis: Vaccine or VariZIG. See Box 5: Post-Exposure Prophylaxis for more details.
- Recommended Vaccines: Two doses of varicella vaccine are recommended for all children, adolescents, and adults without contraindications who do not have evidence of immunity to varicella.
- Reportable Criteria: Report all hospitalized or fatal varicella cases, all varicella cases in high-risk settings, and all outbreaks with 3 or more varicella cases within 1 working day from identification.
Investigation of a Suspected Varicella Case
Investigation of a Suspected Varicella Outbreak
Routine investigation of individual cases of non-fatal, non-hospitalized chickenpox or shingles is not required.
Boxes, Appendix, & Resources
Box 1: Varicella Specimen Collection & Lab Testing
Los Angeles County staff should follow the
Varicella Specimen Collection Standard of Practice MD/ND 151 (Internal Only).
Vesicular or maculopapular lesions or scabs are the preferred method for laboratory confirmation of varicella. Laboratory diagnosis of varicella is not routinely required. However, consider testing for varicella when confirming outbreaks, especially if previously vaccinated cases are experiencing breakthrough disease. In addition, confirmation of hospitalized and fatal varicella cases is required to rule out the rare possibility of smallpox. Serological testing is helpful in confirming current or past disease, or susceptibility to future disease. Clinical and epidemiological history is required to aid the laboratory in test selections. Specimen collection should occur within 3-4 days of rash appearance to maximize the chance of isolating the virus. See table below for a visual depiction of appropriate Specimen Collection Tubes.
A. Polymerase Chain Reaction (PCR)
PCR of scabs or vesicular fluid is the preferred method for laboratory confirmation of varicella. In the absence of vesicles or scabs, scrapings of maculopapular lesions can be collected for testing. Cerebrospinal fluid testing is also acceptable in cases with neurological symptoms.
- Specimen Collection Methods: Video
- Specimen Types: Vesicular skin lesions, maculopapular skin lesions and/or scabs
- Laboratory Form: LAC PHL Test Requisition and Report Form H-3021
- Fill out all required fields.
- Under IMMUNOSEROLOGY/VIROLOGY select "Title 17 Submission (Specify in "Other")".
- Under TITLE 17/OTHER (SPECIFY) type "VZV PCR".
- In addition, work with VPDCP to complete any forms needed by CDPH VRDL.
- Collection/Transport Container: Store swabs from vesicular or maculopapular skin lesions in a Universal Viral Transport Medium (UVTM). Store scabs in a dry, sterile tube.
- Storage: Refrigerate specimens at (2-8°C). Transport refrigerated on cold packs to the laboratory as soon as possible within 48 hours. Do NOT freeze any specimens.
Vesicular Skin Lesions
- Locate skin lesion to perform swab collection. Do not clean lesions with ethanol or any other disinfectant prior to swabbing. Clean area with sterile water or saline, if required.
- Use a sterile polyester swab to collect material from skin lesion surface (do not use swabs with wooden stems).
- Apply firm pressure without causing bleeding and vigorously swab the base of the lesion with the soft end of the applicator swab. If the lesion ruptures while swabbing, ensure the swab collects material and fluid (pus, lymph).
- Place the single swab in the UVTM tube and use scissors to break off the end of the applicator (handle) approximately three (3) inches to ensure the swab fits into the tube. Secure tube top. Only place one swab in each tube to avoid cross-contamination.
- Label tubes individually if specimens are taken from multiple body sites and ensure they are resistant to breakage.
- Place UVTM tube(s) into a biohazard specimen bag.
- Place completed requisition form in outer pouch of biohazard specimen bag.
Maculopapular Skin Lesions
- Rake the edge of the slide over the selected lesion, abrading the lesion with sufficient vigor to ensure that skin cells are gathered onto the slide.
- Use a sterile polyester swab to scrub the abraded lesion and, using the same swab, collect the material collected on the edge of the slide (do not use swabs with wooden stems).
- With young children, it may be less stressful for them if you ask them to help with this.
- Place the single swab in the UVTM tube and use scissors to break off the end of the applicator (handle) approximately three (3) inches to ensure the swab fits into the tube. Secure tube top. Only place one swab in each tube to avoid cross-contamination.
- Label tubes individually if specimens are taken from multiple body sites and ensure they are resistant to breakage.
- Place UVTM tube(s) into a biohazard specimen bag.
- Place completed requisition form in outer pouch of biohazard specimen bag.
Scabs
- Use a glass slide to lift scabs off the skin.
- Transfer scab directly to dry, sterile, break-resistant snap-cap or screw-top tube.
- Label tubes individually if specimens are taken from multiple body sites.
- Place tube(s) into a biohazard specimen bag.
- Place completed requisition form in outer pouch of biohazard specimen bag.
B. Serology for Diagnosis: Paired acute and convalescent sera (IgG)
Note: IgM serology has limited value as a diagnostic method for VZV infection and is not recommended for laboratory confirmation of varicella. However, an IgM positive result in the presence of varicella-like symptoms can indicate a likely acute VZV infection. A positive IgM result in the absence of clinical disease is not considered indicative of active varicella.
- Laboratory Form: LAC PHL Test Requisition and Report Form H-3021
- Fill out all required fields.
- Under IMMUNOSEROLOGY/VIROLOGY select "Varicella IgG Antibody".
- Container: Red or gold top serum separator vacutainer tube, a red-gray top vacutainer tube.
- Specimen Type: Whole clotted blood.
- Amount: 8-10 ml.
- Storage: Refrigerate at 2-8°C. Ship on cold packs within 2 days of sample collection.
- Timing of Specimen Collection: Collect first blood specimen as early as possible and send after it is collected (do not store). Approximately two weeks after collecting the first specimen, collect the second specimen, repeating these steps.
C. Serology to Determine Immunity Status: IgG Test
Submit single blood specimen as outlined above for IgG testing.
| TABLE: Specimen Collection Tubes by Test and Specimen Type | ||
|---|---|---|
| Test, Specimen, Container | Specimen Collection Tube | |
| Test: Varicella zoster virus (VZV) IgG Specimen: Serum Collection/Transport Container: Red or gold-top SST® |
Red or gold-top SST®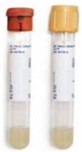 |
|
| Test: Varicella Zoster Virus (VZV) PCR Specimen: Vesicular Lesion Samples or Maculopapular Lesion Samples Collection/Transport Container: Universal Viral Transport Media (UVTM) |
Universal Viral Transport Media (UVTM)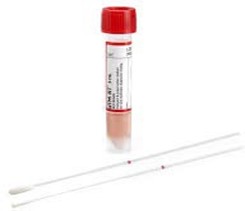 |
|
| Test: Varicella Zoster Virus (VZV) PCR Specimen: Scabs Collection/Transport Container: Sterile Screw-Top or Snap Cap Specimen Tube |
Sterile Screw Cap Specimen Tube |
Sterile Snap Cap Specimen Tube |
Box 2: Presumption of Immunity Criteria
Contacts may be considered immune if they meet any of the following criteria:
- Documentation of age-appropriate varicella vaccination
- For pre-school age children (12 months through 3 years old): 1 dose
- For school-age children, adolescents, and adults: 2 doses
- Laboratory evidence of immunity or lab confirmation of disease*
- Birth in the United States before 1980**
- Diagnosis or verification of a history of varicella and/or herpes zoster by a healthcare provider
*Commercial assays can be used to assess disease-induced immunity, but they lack sensitivity to detect vaccine-induced immunity (i.e., they might yield false-negative results).
**Should not be considered evidence of immunity for healthcare personnel, pregnant people, or immunocompromised people.
Box 3: Severely Immunocompromised Criteria
Severely immunocompromised patients who are exposed to measles should receive IGIV prophylaxis regardless of immunologic or vaccination status because they might not be protected by the vaccine. Per the Infectious Diseases Society of America (IDSA), persons with high-level immunosuppression include those:
- With combined primary immunodeficiency disorder (e.g., severe combined immunodeficiency);
- Who are receiving cancer chemotherapy;
- On treatment for ALL within and until at least 6 months after completion of immunosuppressive chemotherapy;
- Within 2 months after solid organ transplantation;
- Who have received a bone marrow transplant until at least 12 months after finishing all immunosuppressive treatment, or longer in patients who have developed graft-versus-host disease;
- With HIV infection with a CD4 T-lymphocyte count <200 cells/mm3 (age >5 years) and percentage <15 (all ages) (some experts include HIV-infected persons who lack recent confirmation of immunologic status or measles immunity);
- Receiving daily corticosteroid therapy with a dose ≥20 mg (or >2 mg/kg/day for patients who weigh <10 kg) of prednisone or equivalent for ≥14 days; and
- Receiving certain biologic immune modulators, that is, a tumor necrosis factor-alpha (TNF-α) blocker or rituximab.
After HSCT, duration of high-level immunosuppression is highly variable and depends on type of transplant (longer for allogeneic than for autologous), type of donor and stem cell source, and post-transplant complications such as graft vs host disease (GVHD) and their treatments.
Box 4: Risk Level Assessment
High-risk contacts are those who may experience severe illness if infected or to whom transmission potential is high. High-risk contacts who self-report varicella immunity have 24 hours from initial interview to produce evidence of immunity (see Box 2). As needed, coordinate rapid serologic testing for varicella immunity (varicella IgG) for high-risk contacts whose varicella immune status is not known.
Examples of high-risk individuals:
- Pregnant people
- Severely immunocompromised people (see Box 3)
- Household contacts
- People who work in high-risk settings. High-risk settings include locations in which transmission risk is high (e.g., healthcare setting or other congregate setting). Healthcare contacts who are not healthcare workers and are not otherwise high risk can be followed up as low risk.
Low-risk contacts are those who are not at high risk of experiencing severe illness and to/from whom transmission potential is not high.
Examples of low-risk individuals:
- Immunocompetent
- Not pregnant
- Not a healthcare worker
- Not a household contact
Box 5: Post-Exposure Prophylaxis (PEP)
VariZIG: Varicella zoster immune globulin (VariZIG) should be administered as soon as possible and no later than 10 days after first date of exposure for any contacts at high risk of severe varicella infection (see Figure 1).
- To obtain VariZIG, contact the DPH pharmacy at 213-250-8616 or the distributor FFF Enterprises at their 24-hour telephone number: 1-800-843-7477.
Varicella Vaccine: A dose of varicella-containing vaccine as PEP may be effective in preventing or modifying illness severity if given within 3-5 days after the first date of exposure.
- Contacts with No Varicella Vaccine Doses: A first dose of varicella-containing vaccine should be given within 5 days after the first date of exposure to patients who do not have contraindications. Also assess patient for precautions before administering vaccine.
- Contacts with One Dose of Varicella Vaccine: A second dose of varicella vaccine can be given to patients who do not have contraindications:
- Children <13 years of age can receive a second dose 3+ months after their first dose.
- People ≥ 13 years old can receive a second dose 4+ weeks after their first dose.
Note: Inform patients that some contacts may have been exposed at the same time as the index case and that the vaccine may not protect against disease in this circumstance. Varicella-containing vaccines should not be used to immunize women who are pregnant or who intend to become pregnant within one month.
Box 6: Case Definition (CDC, 2024-Present)
Clinical Criteria
In the absence of a more likely alternative diagnosis:
- An acute illness with a generalized rash with vesicles (maculopapular vesicular rash), OR
- An acute illness with a generalized rash without vesicles (maculopapular rash).
Laboratory Criteria
Confirmatory Laboratory Evidencea
- Positive polymerase chain reaction (PCR) for varicella-zoster virus (VZV) DNAb,c, OR
- Positive direct fluorescent antibody (DFA) for VZV DNA, OR
- Isolation of VZV, OR
- Significant rise (i.e., at least a 4-fold rise or seroconversion c,d) in paired acute and convalescent serum VZV immunoglobulin G (IgG) antibodyc,e.
Supportive Laboratory Evidence:
- Positive test for serum VZV immunoglobulin M (IgM) antibody c,f
Note: The categorical labels used here to stratify laboratory evidence are intended to support the standardization of case classifications for public health surveillance. The categorical labels should not be used to interpret the utility or validity of any laboratory test methodology.
- A negative laboratory result in a person with a generalized rash with vesicles does not rule out varicella as a diagnosis.
- PCR of scabs or vesicular fluid is the preferred method for laboratory confirmation of varicella. In the absence of vesicles or scabs, scrapings of maculopapular lesions can be collected for testing.
- Not explained by varicella vaccination during the previous 6-45 days.
- Seroconversion is defined as a negative serum VZV IgG followed by a positive serum VZV IgG.
- In vaccinated persons, a 4-fold rise may not occur.
- IgM serology has limited value as a diagnostic method for VZV infection and is not recommended for laboratory confirmation of varicella. However, an IgM positive result in the presence of varicella-like symptoms can indicate a likely acute VZV infection. A positive IgM result in the absence of clinical disease is not considered indicative of active varicella
Epidemiologic Linkage
Confirmatory Epidemiologic Linkage Evidence:
- Exposure to or contact with a laboratory-confirmed varicella case, OR
- Can be linked to a varicella cluster or outbreak containing ≥1 laboratory-confirmed case, OR
- Exposure to or contact with a person with herpes zoster (regardless of laboratory confirmation).
Presumptive Epidemiologic Linkage Evidence:
- Exposure to or contact with a probable varicella case that had a generalized rash with vesicles.
Case Classification
Probable:
- Meets clinical evidence with a generalized rash with vesicles,
OR - Meets clinical evidence with a generalized rash without vesicles AND:
- Confirmatory or presumptive epidemiologic linkage evidence, OR
- Supportive laboratory evidence.
OR
- Meets healthcare record criteria* AND:
- Confirmatory or presumptive epidemiologic linkage evidence, OR
- Confirmatory or supportive laboratory evidence.
*A person whose healthcare record contains a diagnosis of varicella or chickenpox but no rash description.
Confirmed:
Meets clinical evidence AND confirmatory laboratory evidence,
OR
Meets clinical evidence with a generalized rash with vesicles AND confirmatory epidemiologic linkage evidence.
Death Classification:
Confirmed: A death resulting from a confirmed case of varicella which contributes directly or indirectly to acute medical complications that result in death.
Probable: A death resulting from a probable case of varicella which contributes directly or indirectly to acute medical complications that result in death.
Box 7: Contraindications and Precautions to Varicella Vaccination
Contraindications:
- Severe allergic reaction to vaccine component or following a prior dose
- Immunosuppression due to leukemia, lymphoma, generalized malignancy, immune deficiency disease, or immunosuppressive therapy
- Family history of congenital or heredity immunodeficiency in first-degree relatives
- HIV infection*
- Hematopoietic stem cell transplant (wait 24 months)
- Pregnancy
*Contraindicated for MMRV; contraindicated for VAR depending on CD4 count
Precautions:
- Moderate or severe acute illness
- Alpha-gal allergy (consult with physician)
- Receipt of antibody-containing blood products (wait 3 to 11 months to vaccinate)
- Need for tuberculosis testing (precaution for MMRV only)
- Receipt of specific antiviral drugs 24 hours before vaccination
- Simultaneous use of aspirin or aspirin-containing products
- Personal or family history of seizures of any etiology (precaution for MMRV only)
Figure 1: Management of Exposures to Varicella-Zoster Virus
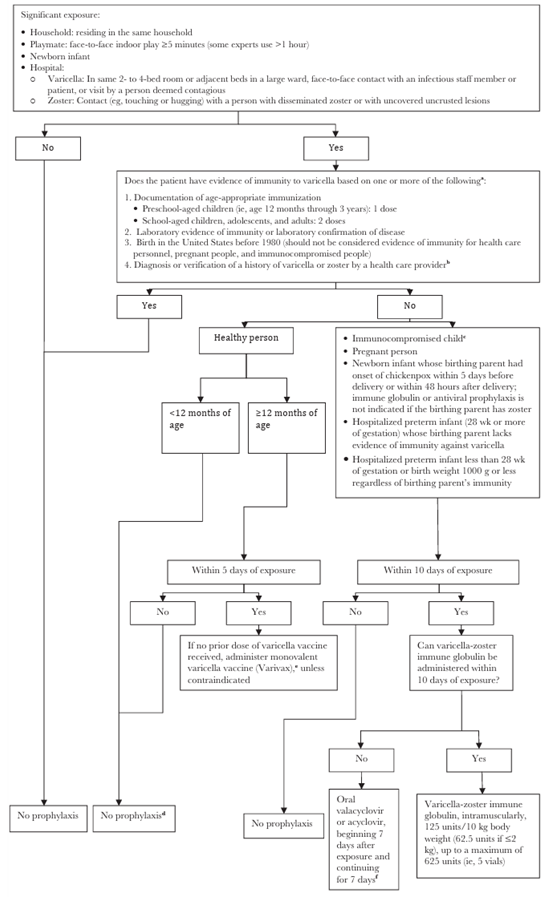
IGIV indicates immune globulin intravenous.
a People who receive hematopoietic cell transplants should be considered nonimmune regardless of previous history of varicella disease or varicella vaccination in themselves or in their donors.
b To verify a history of varicella in an immunocompromised child, health care providers should inquire about an epidemiologic link to another typical varicella case or to a laboratory confirmed case, or evidence of laboratory confirmation. Immunocompromised children who have neither an epidemiologic link nor laboratory confirmation of varicella should not be considered as having a valid history of disease.
c Immunocompromised children include those with congenital or acquired T-lymphocyte immunodeficiency, including leukemia, lymphoma, and other malignant neoplasms affecting the bone marrow or lymphatic system; children receiving immunosuppressive therapy, including ≥2 mg/kg/day of systemic prednisone (or its equivalent) for ≥14 days, and certain biologic response modifiers; all children with human immunodeficiency virus (HIV) infection regardless of CD4+ T-lymphocyte percentage; and all hematopoietic cell transplant patients regardless of pretransplant immunity status.
d If the exposed person is an adolescent or adult, has chronic illness, or there are other compelling reasons to try to avert varicella, some experts recommend preemptive therapy with oral valacyclovir or acyclovir (see Chemoprophylaxis, below, for dosing). For exposed people ≥12 months of age, vaccination is recommended for protection against subsequent exposures.
e If 1 prior dose of varicella vaccine has been received, a second dose should be administered at ≥4 years of age. If the exposure occurred during an outbreak, a second dose is recommended for preschool-aged children younger than 4 years for outbreak control if at least 3 months have passed after the first dose.
f See Chemoprophylaxis, below, for dosing. If varicella-zoster immune globulin and either valacyclovir or acyclovir are not available, IGIV may be administered (400 mg/kg).
Chemoprophylaxis: For immunocompromised patients without evidence of immunity or for immunocompetent patients for whom varicella prevention is desired (e.g., healthy older adolescent or adult contacts for whom vaccination is not possible) who have been exposed to varicella or herpes zoster, valacyclovir (20 mg/kg per dose, administered orally 3 times per day, with a maximum daily dose of 3000 mg), if available and dosage form is tolerable, or acyclovir (20 mg/kg per dose, administered orally 4 times per day, with a maximum daily dose of 3200 mg) may be used as chemoprophylaxis if passive immunoprophylaxis is not utilized. Antiviral treatment should begin 7 days after exposure and should continue for a total of 7 days. No studies of oral prophylaxis have been performed for adults. VZV-seropositive patients receiving intensive and/or myeloablative chemotherapy should routinely receive antiviral prophylaxis; children receiving antiviral prophylaxis or treatment with valganciclovir, ganciclovir, or foscarnet do not require additional antiviral prophylaxis against VZV.
Resources:
Varicella Notification Letters and Flyers:
- Exposure Notification for Healthcare Staff Exposed to Varicella:
- Exposure Notification for Non-Healthcare Exposed to Varicella:
- Facility Notification of Varicella Exposure:
- DPH Only:
- Healthcare Facility (English)
- Healthcare Facility (Spanish)
- Non-Healthcare Facility (English)
- Non-Healthcare Facility (Spanish)
- Public/Provider:
- Healthcare Facility (English (coming soon))
- Healthcare Facility (Spanish (coming soon))
- Non-Healthcare Facility (English (coming soon))
- Non-Healthcare Facility (Spanish (coming soon))
- Not Exposed to Varicella Notification:
- Off Work Notice (DPH Only)
- End of Varicella Situation:
Varicella Reporting Forms:
- Varicella Death Investigation Worksheet (VPDC to Complete and Send)
- Varicella Death Investigation Worksheet Instructions
- Varicella Hospitalization or Death Case Report Form (VPDC to Complete and Send)
- Varicella Line List for Outbreak Sites
- Varicella Outbreak Surveillance Reporting Line List (2024 Version)
- Varicella Outbreak Surveillance Reporting Line List Instructions (2024 Version)
Varicella Vaccine Recommendations:
- Measles, Mumps, Rubella, and Varicella Vaccine (MMRV) (CDPH)
- Recommended Child and Adolescent Immunization Schedule for Ages 18 Years or Younger (AAP)
Other Varicella Resources:
- Chickenpox (Varicella) Information for Public & Providers (LACDPH)
- How to Classify Confirmed & Probable Varicella Cases During Investigations (CDC)
- Infection Control in Healthcare Personnel: Epidemiology and Control of Selected Infections Transmitted Among Healthcare Personnel and Patients (CDC)
- Update to Public Health Reporting and National Notification of Varicella (CSTE)
- Varicella Quicksheet (CDPH)
- Zoster (Shingles) Quicksheet (CDPH)
Acute Communicable Disease Control Manual (B73):

