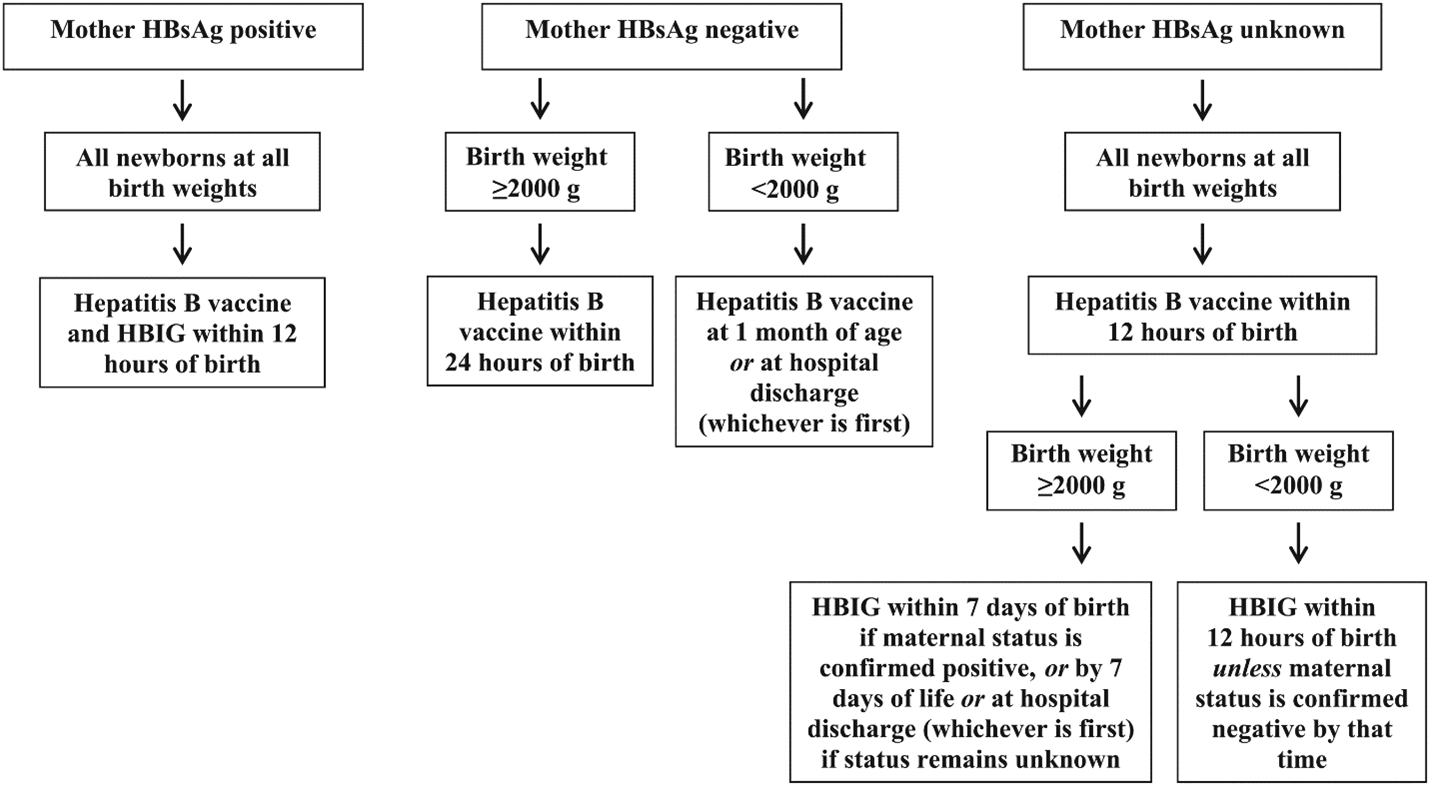
- AAP recommends infants born to HBsAg-positive persons receive single antigen dose of hepatitis B vaccine (Engerix-B or Recombivax HB) and hepatitis B immune globulin (HBIG) within 12 hours of birth, regardless of birthweight or any maternal antenatal treatment with antiviral medications.
- Infants born to HBsAg-positive persons should complete the hepatitis B vaccination series to ensure protection against hepatitis B. For infants with birthweight < 2000 gm, a 4-dose series is needed; for infants with birthweight ≥ 2000 gm, a 3-dose series is needed.
- Administer the final dose no earlier than 6 months of age (minimum age of 164 days includes 4-day grace period).
Post-Vaccination Serologic Testing
- Post-vaccination serologic testing (PVST) helps identify if an infant born to an HBsAg-positive person has an adequate immune response to an initial hepatitis B vaccine series. If they do not, the infant may require additional vaccination. PVST can also identify infants with HBV infection.
- After completion of the hepatitis B infant vaccine series, perform PVST on infants 9-12 months of age or 1-2 months after completion of at least 3 doses of a hepatitis B vaccination series, whichever occurs latest.
- PVST is only recommended for infants who were exposed to hepatitis B during birth and have completed their full 3- or 4-dose
infant vaccine series. It should not be performed on infants who are not exposed or before the completion of the full series of vaccine.
A positive or a negative antibody test after 1 or 2 doses of vaccine is of unknown clinical significance and should not inform whether a child needs additional doses without
peer-reviewed published studies evaluating the long-term efficacy of an alternative dosing schedule in infants.
Request the following labs:
- HBsAg (Hepatitis B Surface Antigen)
- Anti-HBs, quantitative (Hepatitis B Surface Antibody)
- NOTE: Antibody to hepatitis B core antigen (anti-HBc) testing of infants is not recommended because passively acquired maternal anti-HBc might be detected in infants born to a person who is HBsAg-positive up to age 24 months.
- HBsAg-negative infants with anti-HBs levels ≥10 mIU/mL after their full 3- or 4-dose series
are protected and need no further medical management.
- HBsAg-negative infants with anti-HBs <10 mIU/mL should be revaccinated with a single dose of hepatitis B vaccine and receive a second round of PVST 1–2 months later. Infants whose anti-HBs remains <10 mIU/mL following the single dose revaccination should then receive two more doses of hepatitis B vaccine to complete the second series, followed by a third round of PVST 1–2 months after the final dose.
- For more information, see California Department of Public Health guidelines for pediatric providers to prevent chronic hepatitis B in children.